In this project we employ computer simulation techniques to improve the fundamental understanding of how electromagnetic radiation interacts with biological matter.
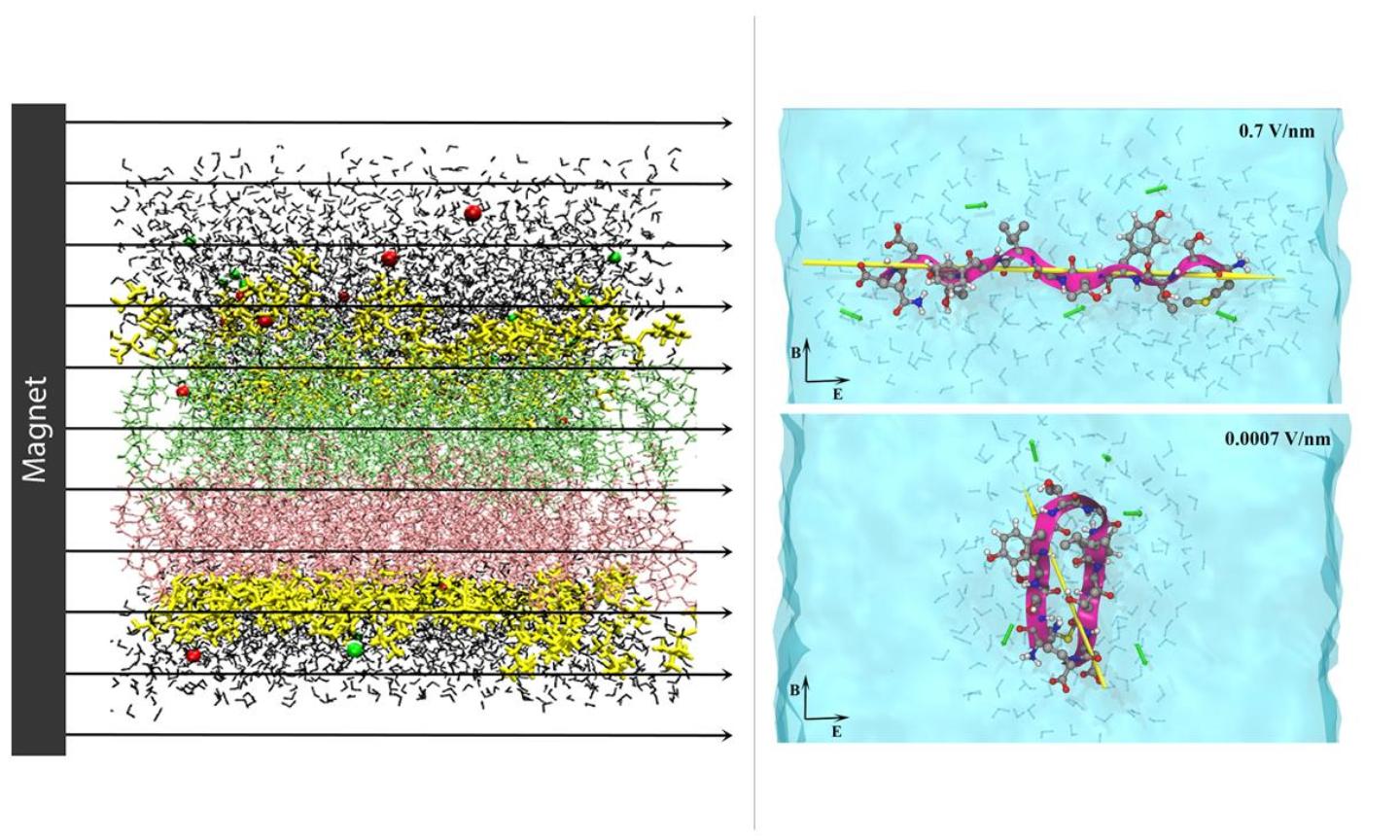 Molecular model of a lipid membrane in magnetic field (left); Peptide model in solution under electromagnetic field (right).
Molecular model of a lipid membrane in magnetic field (left); Peptide model in solution under electromagnetic field (right).
The world's population is increasingly exposed to new physical and chemical agents in the environment, some of which may be beneficial and others, damaging to health. Among these new agents, electromagnetic fields (EMF) are becoming more widespread, and their application in new technologies continues to grow, with novel uses being actively developed and commercialised. In particular, the electromagnetic (EM) energy that powers mobile technologies is now affecting over 5 billion phone subscribers world-wide, so the community concern about the possibility of associated health effects is also growing. The mechanisms of non-thermal EMF effects on biological systems, such as proteins and membranes, are not well understood, however, it is believed that EMFs (at certain intensities and frequencies) can cause structural changes which can alter molecular conformation and ultimately the function of biological molecules.
Our team conducts fundamental studies of the molecular structure and dynamics of proteins, peptides and biological membranes exposed to external non-ionising electromagnetic fields typically produced by mobile electronic devices. The latest techniques in computational modelling of biomolecules at all-atom resolution will be employed to explore the molecular mechanisms of their response to the electromagnetic radiation. The studies will help understand potential health implications of mobile technologies and inform safety standards for their manufacturing.
Grants and funding
Funded by the NHMRC Center of Research Excellence “Australian Center for Electromagnetic Bioeffects Research” (ACEBR) 2013-2022. where our team employs computer modelling techniques to study the molecular level response of proteins and biological membranes to electromagnetic radiation in order to develop a fundamental understanding of possible health implications of the widespread use of mobile and wireless communication devices.
Key people
- Professor Irene Yarovsky, Chief Investigator and team leader
- Dr Nevena Todorova, Senior Lecturer
- Dr Harun Rashid, Research Fellow (2014 - 2018)
- Alan Bentvelzen, MSci, completed 2018
- Dr. Ben Noble, Research Fellow
Partners
- Dr Niall English, University College Dublin, Ireland
- Professor Boris Martinac, Victor Chang Cardiac Research Institute
- Professor Elena Ivanova, Physics, RMIT University
- Effects of electromagnetic field characteristics on conformational response of an amyloidogenic peptide, A. Bentvelzen, N. Todorova, I. Yarovsky, J Chem Phys, 2020
- The enigma of amyloid forming proteins: challenges and insights from molecular simulations, N. Todorova and I. Yarovsky, Australian Journal of Chemistry, invited review, RACI Awards special issue, 72 (2019) 574-584
- Residue-Specific Solvation Directed Thermodynamic and Kinetic Control over Peptide Self-Assembly with 1D/2D Structure Selection, Y. Lin, M. Penna, M. R. Thomas, V. Leonardo, Y. Wang, E. T. Pashuck, I. Yarovsky, M. M. Stevens, ACS Nano, 13 (2019) 1900-1909
- Surface Dynamics and Ligand-Core Interactions of Quantum Sized Photoluminescent Gold Nanoclusters, Y. Lin, P. Charchar, A. J. Christofferson, M. R. Thomas, N. Todorova, M.M. Mazo, Q. Chen, J. Doutch, R. Richardson, I. Yarovsky, M. M. Stevens, J. Am. Chem. Soc., 140 (2018) 18217-18226
- Polymorphism in disease-related apolipoprotein C-II amyloid fibrils: a structural model for rod-like fibrils, C. Zlatik, Y. Mao, N. Todorova, Y.F. Mok, G. Howlett, I. Yarovsky, P. Gooley, M. Griffin, FEBS Journal, 285 (2018) 2799–2812
- Effects of forcefield and sampling method in all-atom simulations of inherently disordered proteins: application to conformational preferences of human amylin, E. Peng, N. Todorova, I. Yarovsky, PLoS ONE, 12(10), 2017, e0186219
- Intra- and inter-subunit charge-pair interactions determine the ability of apolipoprotein C-ll mutants to form hybrid amyloid fibrils, N. Todorova, C. O. Zlatic, Y. Mao, I. Yarovsky, G. J. Howlett, P. R. Gooley, and M. D. W. Griffin, Biochemistry, 56 (2017) 1757–1767
- Solution conditions affect the ability of K30D mutation to prevent the amyloid fibril formation by apolipoprotein C-II: insights from experiments and theoretical simulations, Y. Mao, N. Todorova, C. O. Zlatic, P. R. Gooley, M.D. W. Griffin, G. J. Howlett and I. Yarovsky, Biochemistry, 55 (2016) 3815-3824
- S. P. Loughran, Md S. Al Hossain, A. Bentvelzen, M. Elwood, J. Finnie, J. Horvat, S. Iskra, E. P. Ivanova, J. Manavis, C. K. Mudiyanselage, A. Lajevardipour, B. Martinac, R. McIntosh, R. McKenzie, M. Mustapic, Y. Nakayama, E. Pirogova, M. H. Rashid, N. A. Taylor, N. Todorova, P. M. Wiedemann, R. Vink, A. Wood, I. Yarovsky, R. J. Croft “Bioelectromagnetics Research within an Australian Context: The Australian Centre for Electromagnetic Bioeffects Research (ACEBR)” International Journal of Environmental Research and Public Health, 3(10), 967 (2016)
- N. Todorova, A. Bentvelzen, N. J. English, I Yarovsky, "Electromagnetic-field effects on structure and dynamics of amyloidogenic peptides" J. Chem. Phys. 144(8): 085101 (2016)
- A. Budi, F.S. Legge, H. Treutlein, I. Yarovsky, "Comparative Study of Insulin Chain-B in Isolated and Monomeric Environments under External Stress" J. Phys. Chem. B, 112(26):7916-7924 (2008)
- A. Budi, F.S. Legge, H. Treutlein, I. Yarovsky, "Effect of Frequency on Insulin Response to Electric Field Stress" J. Phys. Chem. B, 111(20):5748-5756 (2007)
- F.S. Legge, A. Budi, H. Treutlein, I. Yarovsky, "Protein Flexibility: Multiple Molecular Dynamics Simulations of Insulin Chain B" Biophys. Chem., 119(2):146-157 (2006)
- A. Budi, F.S. Legge, H. Treutlein, I. Yarovsky, "Electric Field Effects on Insulin Chain-B Conformation" J. Phys. Chem. B, 109(47):22641-22648 (2005)
- A. Budi, F.S. Legge, H. Treutlein, I. Yarovsky, "Protein Response to Electric Field Stress" Proceedings of the 16th Australian Institute of Physics Congress 2005 Canberra, Australia (2005) ISBN 0-9598064-8-2
- A. Budi, F.S. Legge, H. Treutlein, I. Yarovsky, "Effect of External Stresses on Protein Conformation: A Computer Modelling Study" Eur. Biophys. J., 33(2):121-129 (2004)


