The aims of this project are to synthesise new carbohydrate-based surfactants optimised for use as cryoprotectants, and to accurately measure, model and optimise their performance.
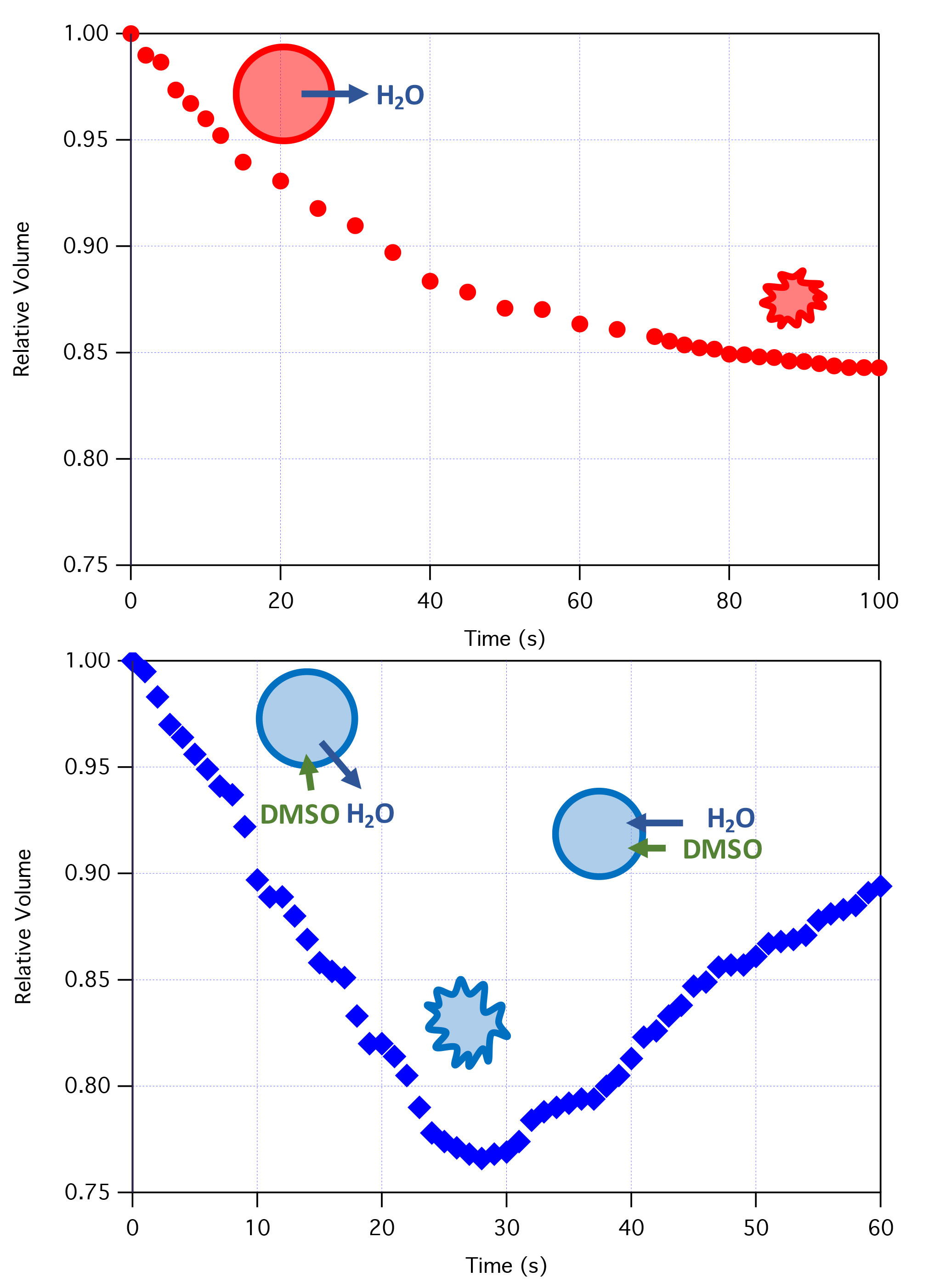 Figure 1. Volume response of cells upon the addition of non-penetrating (top) or penetrating (bottom) solutes.
Figure 1. Volume response of cells upon the addition of non-penetrating (top) or penetrating (bottom) solutes.
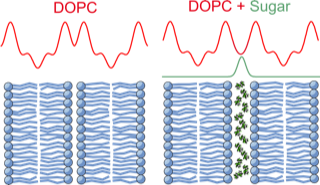 Figure 2. Scattering length density profiles of membranes with and without additional solutes. The detailed location of solutes within membrane systems can be obtained by Neutron and X-ray scattering methods.
Figure 2. Scattering length density profiles of membranes with and without additional solutes. The detailed location of solutes within membrane systems can be obtained by Neutron and X-ray scattering methods.
Project title: Rational design of new synthetic antifreeze molecules for cryopreservation
Project dates: 2019 – 2022
Grants and funding: ARC Discovery DP190101010
Description
The aims of this project are to synthesise new carbohydrate-based surfactants optimised for use as cryoprotectants, and to accurately measure, model and optimise their performance. An international, interdisciplinary research team will use state of the art experimental methods and advanced phase-field modelling techniques to optimise the cryoprotectants so that they reduce osmotic stress in cells and inhibit ice crystal growth during freezing and thawing. The expected outcomes will be novel cryoprotectants that are easy to synthesise, non-toxic and effective, opening up new possibilities for the cryopreservation of cells, organs and possibly even whole organisms.
Research strategy
There are three interlocked research themes:
Measurement: Understanding the biophysical properties of the novel molecules by determining their thermal behaviour (differential scanning calorimetry), their membrane permeability (live cell permeability assays) and their interaction with membranes (Langmuir monolayer studies and X-ray and Neutron scattering studies).
Synthesis: We will target two general classes of compounds based on existing molecules with known cryoprotective properties: trehalose and sucrose esters by varying of substitution degree and pattern to improve cell permeability.
Modelling: Phase field modelling of the molecules to accurately and quantitatively predict how differences in molecular structure are reflected in changes in the dynamical processes important for cryopreservation.
Rationale
Since the addition of glycerol was first used for cryopreservation in 1949, cryopreservation methodologies based on the addition of cryoprotectants (CPAs) have evolved to the point that we can now successfully cryopreserve red and white blood cells, sperm, embryos and some cancer and stem cells . Cryopreservation has also been used for the storage of simple organelles such as seeds and shoot tips, and for the long-term preservation of endangered species of plants and animals.
Current methods have two significant disadvantages: i) the CPAs used are highly toxic, and must be rapidly removed upon thawing, which is expensive and time consuming - this is far from ideal for critical applications such as reproductive technologies, preservation of endangered plant and animal species and stem cell therapies; and ii) despite years of research, most cell types (>hundreds) cannot be cryopreserved using existing CPAs.
The aims of this project are:
- To quantify the critical molecular properties that lead to good cryoprotective performance.
- To use this knowledge to design new, non-toxic carbohydrate derivatives optimised for use as CPAs.
Key people
- Professor Gary Bryant
- Professor Peter Daivis
- Dr. Saffron Bryant
External:
- Dr Brendan Wilkinson (University of New England)
- Professor Ken Elder (Oakland University, USA)
Associated journal publications
- Raju, R.; Merl, T.; Adam, M. K.; Staykov, E.; Ben, R. N.; Bryant, G.; Wilkinson, B. L., n-Octyl (Thio)glycosides as Potential Cryoprotectants: Glass Transition Behaviour, Membrane Permeability, and Ice Recrystallization Inhibition Studies. Aust. J. Chem. 2019, 72 (8), 637-643. DOI:/10.1071/CH19159
- Raju, R.; Hohn, H.; Karnutsch, C.; Khoshmanesh, K.; Bryant, G., Measuring volume kinetics of human monocytes in response to cryoprotectants using microfluidic technologies. Applied Physics Letters 2019, 114, 223702. DOI:/10.1063/1.5096199
- Hannam, S. D. W.; Daivis, P. J.; Bryant, G., Compositional relaxation on the approach to the glass transition in a model trehalose solution. Phys. Rev. E 2019, 99 (3), 032602. DOI:/10.1103/PhysRevE.99.032602


