Chathumini Samarawickrama
Atmospheric corrosion & prevention are widely studied fields all around the world due to the significant costs associated with maintenance against corrosion. Structural metals such as steel & galvanised steel are widely used in the automotive & construction industries, which has led to their corrosion being extensively studied. Corrosion protection of these metals is most commonly achieved via protective coatings and/or corrosion inhibitors. However, environmental legislation and the necessity for sustainable solutions now drive the need for environmentally friendly corrosion inhibitors. Numerous studies have been undertaken to determine the performance & mechanism of a wide range of organic and non-organic inhibiting compounds. Whilst most studies were conducted utilizing a bulk volume of electrolyte, only limited research has studied corrosion protection mechanisms associated with droplets of various volumes. In addition to atmospheric corrosion studies, with cabinet methods such as salt spray tests, it is also crucial to focus on minute volumes to represent aerosols of aqueous droplets deposited on metal surfaces. In contrast to a bulk electrolyte environment, droplet volumes & shapes, electrolyte [1] evaporation, secondary spreading, and diffusion gradients must be accounted for alongside corrosion measurements. Therefore, this research study will focus on the differences in the effectiveness & mechanisms of corrosion-inhibiting compounds in droplet-sized volumes vs. bulk corrosion under a bulk volume of electrolyte. The influence of droplet volume, pH, humidity, salt load density, etc. on inhibitor performance for a wide range of inhibiting compounds will be elucidated via utilization of electrochemical & surface analytical techniques.
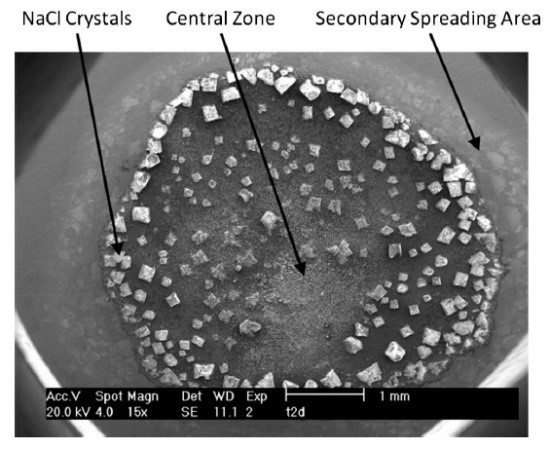 Figure 1: Corrosion products formed during the interaction of seawater droplets on zinc surface [1]
Figure 1: Corrosion products formed during the interaction of seawater droplets on zinc surface [1]
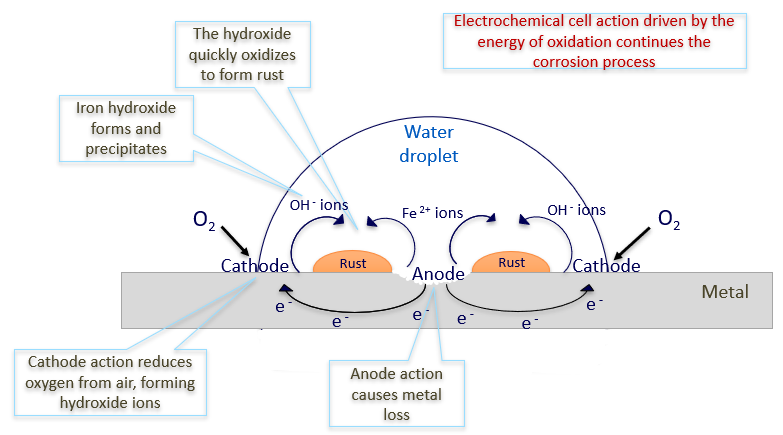 Figure 2: Schematic illustration of corrosion of iron under an electrolyte droplet’s cross section [2]
Figure 2: Schematic illustration of corrosion of iron under an electrolyte droplet’s cross section [2]
This project is conducted in conjunction with BASF Coatings.
References
[1] I Cole, T Muster, D Lau, N Wright, NS Azmat (2010). ‘Products formed during the interaction of seawater droplets with zinc surfaces: II. Results from short exposures’, Journal of the Electrochemical Society, vol. 157, no. 6, p. C213, DOI: 10.1149/1.3391383.
[2] BG Koushik, N Van den Steen, MH Mamme, Y Van Ingelgem, H Terryn (2021). ‘Review on modelling of corrosion under droplet electrolyte for predicting atmospheric corrosion rate’, Journal of Materials Science and Technology, review vol. 62, pp. 254-267, DOI: 10.1016/j.jmst.2020.04.061.

Corrosion and Inhibition
Corrosion inhibitors, corrosion of steel pipes in a soil environment, photocatalysts for CO2 hydrogenation, nano-sensing, optical sensing.