José María Castillo Robles
Corrosion is a major problem in the industry, and to mitigate it, toxic inorganic compounds are often used as corrosion inhibitors. However, over the last few years, they are being replaced by more environmentally friendly compounds. Nonetheless, the fundamental mechanism determining their inhibition performance are still far from understood. Computational simulations can provide us with important insights into those mechanisms allowing for a detailed analysis of the metallic-inhibitor-aqueous interface. Nonetheless, the actual simulations reported in the literature present some main gaps in their works, such as 1) the absence of the electrode potential effect in the metallic surface; 2) absence of solvent effects; 3) current DFT models being limited in scale and function; 4) and the absence of cathodic and anodic reactions. To address precisely those gaps, a multiscale approach is used to address this problem by combining density functional theory (DFT) and non-equilibrium Green’s functions (NEGF) formalism coupled with the quantum mechanics/molecular mechanics (QM/MM) method. Specifically, the interaction between corrosion inhibitors and metallic surfaces, and how the aqueous solution affects that, will be investigated. The first corrosion inhibitor that will be considered is the 2-mercaptobenzimidazole (MBI), known as an effective inhibitor for Cu. As a starting point, Cu (111) will be considered as the metallic surface. After that, the calculations will be extended to more complex surface models, including oxides (Cu2O) and Al alloys, and other corrosion inhibitors.
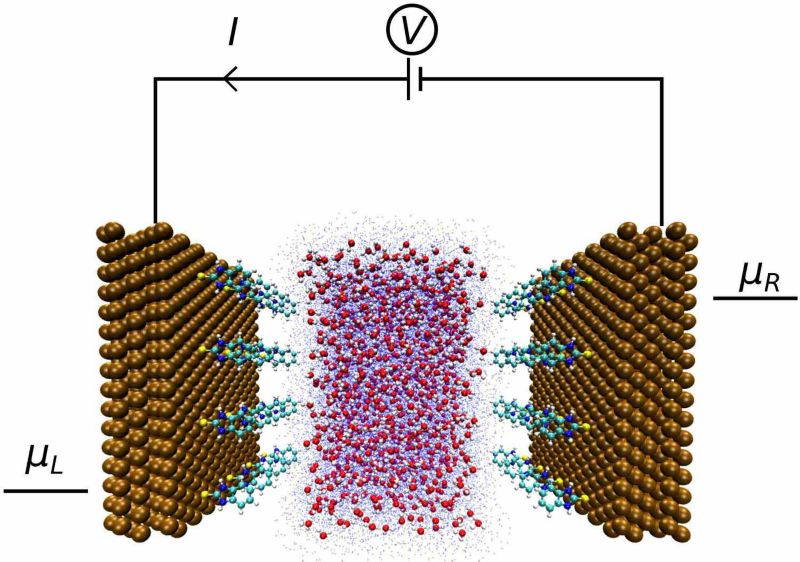
An innovative approach for modelling electrochemistry processes from first principles is through a setup consisting of two electrodes acting as charge reservoirs, which are kept at different potentials, that are separated by an aqueous electrolyte. This setup is widely used to simulate electronic transport in nano-devices, where electrodes held at different potentials are connected to a nano-device through which an electric current is established. The method, first described in the context of DFT in [1] and now implemented in the TranSIESTA code [1,2] (part of the open-source SIESTA software [3,4]), is now a standard for simulation of electronic transport in electronic nano-devices. To enable the use of realistic electrolyte solutions, we will apply the QM/MM method [5,6], in which the system is split into quantum and classical regions according to the chemical processes of interest. This domain decomposition allows us to include in the QM region a reduced number of atoms (the metallic surface + the adsorbed molecules) with the remaining atoms (aqueous solution) modelled in the MM region.
José María is cotutelle with the Autonomous University of Barcelona (UAB) & the Catalan Institute of Nanoscience and Nanotechnology (ICN2, Spain).
References
[1] Brandbyge. M., et al. (2002). ‘Density-functional method for nonequilibrium electron transport’, Physical Review B, 65, 165401, DOI: 10.1103/physrevb.65.165401.
[2] Papior. N., et al. (2017). ‘Improvements on non-equilibrium and transport Green function techniques: The next-generation TRANSIESTA’, Computer Physics Communications, 212, 8-24, DOI: 10.1016/j.cpc.2016.09.022.
[3] Soler. J.M., et al. (2002). ‘The SIESTA method for ab initio order-N materials simulation’, Journal of Physics: Condensed Matter, 14, 2745, DOI: 10.1088/0953-8984/14/11/302.
[4] Garcia. A., et al. (2020). ‘Siesta: Recent developments and applications’, Journal of Chemical Physics, 152, 204108, DOI: 10.1063/5.0005077.
[5] Ordejón. P. (2020). ‘Addressing electrified water-metal interfaces with Non-Equilibrium Green's Functions’, APS March Meeting, F45.00001.
[6] Sanchez-Navarro. C., et al. (2011). ‘An efficient implementation of a QM–MM method in SIESTA’. Theoretical Chemistry Accounts, 128, 825-833, DOI: 10.1007/s00214-010-0816-5.
José María Castillo Robles is cotutelle with the Autonomous University of Barcelona (UAB) & the Catalan Institute of Nanoscience and Nanotechnology (ICN2, Spain).

Corrosion and Inhibition
Corrosion inhibitors, corrosion of steel pipes in a soil environment, photocatalysts for CO2 hydrogenation, nano-sensing, optical sensing.


