Vijay Sisarwal
Implantable medical devices, such as orthopaedic devices, trauma devices, cardiac valves, pacemakers, dental implants, cardiac implants, and coronary stents may all suffer from implant-related infections, leading to failure and high associated economic & social costs. The literature defines the most critical pathogenic event during infection is biofilm formation, which starts immediately after bacterial adhesion on an implant and effectively protects the microorganisms from the immune system and systemic antibiotics. A sound rationale for the prevention of biomaterial-associated infections should thus focus specifically on the inhibition of both bacterial adhesion & biofilm formation. However, prevalent antibacterial strategies consisting of organic & inorganic biocides incur significant concerns with respect to the safety of the patient’s healthy cells & organs.
This PhD project aims to develop a natural biocide-based coating (with low toxicity to bone cells) for countering bacterial strain adhesion and the subsequent formation of biofilms upon the implant surfaces. Strontium, which is a key player in facilitating bone formation, will be combined into the coating system to provide the additional benefit of accelerated bone formation. Several coating techniques will be explored to identify optimal ways to produce a composite coating system upon the surface of implant materials, which may be made from metal, polymer, or ceramic.
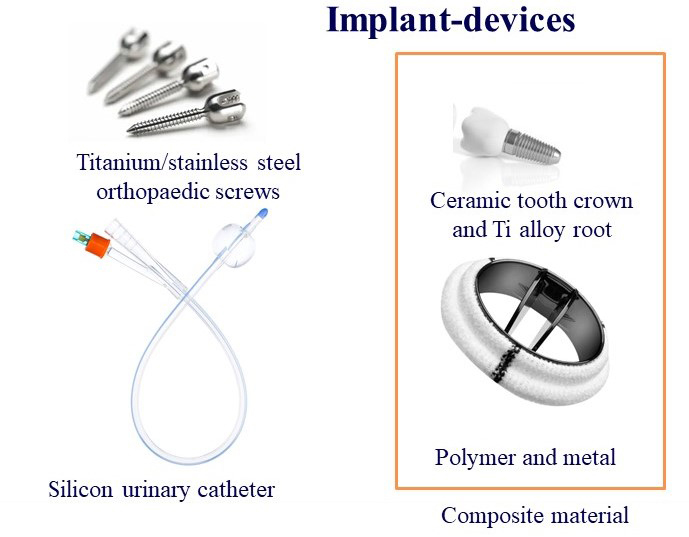 Figure 1: Implantable devices are made of biomaterials that are broadly classified into metals, polymers, ceramics, and composites
Figure 1: Implantable devices are made of biomaterials that are broadly classified into metals, polymers, ceramics, and composites
Vijay Sisarwal is supported by a Government of India scholarship.
Project Publications
Sisarwal, V., Dong, S., Toh, R.J., Gamaleldin, K., Kulkarni, S., Li, H., Cole, I.S., Dong, J., and Chen, X. (2022). ‘Plasma electrolytic oxidation upon Mg alloys: fundamentals, state-of-the-art progress and challenges’, pp 445-464 in: Saji, V.S., Sankara Narayanan, T.S.N., Chen, X. (eds) ‘Conversion Coatings for Magnesium and its Alloys’, Springer, Cham. DOI: 10.1007/978-3-030-89976-9_20.

Metal Fabrication
Cold spray, melt pool, friction stir welding, multifunctional coatings for biomedical Mg alloys, visual monitoring of metal powder